Basketball Board,Pc Basketball Board,Portable Basketball Board,Adjustable Pc Basketball Board skyshields(suzhou)thermoforming tech.co.ltd , https://www.skyshields.com
When infrared light passes through a sample of an organic compound, its wavelengths at some frequencies are absorbed while other wavelengths penetrate the sample without being absorbed. If we plot absorbance or transmittance versus frequency or wavelength, we get an infrared spectrum. For a nonlinear molecule (with n atoms), three degrees of freedom are used to describe its rotation, and three degrees of freedom are used to describe its translation. The remaining 3n-6 degrees of freedom are used to describe the degree of freedom or basic vibration. In addition linear molecules have 3n-5 vibrational degrees of freedom. In addition to the basic vibration, the wavelength of other frequencies is mainly obtained by modulation. Not all peaks in the spectrum are important in the analysis, only those characteristic peaks are of primary interest to us.
The instrument is based on the measurement of the infrared frequency and the infrared spectrophotometer is mainly divided into two basic types. The first is the natural scattering type and the other is the interferometer type. In the former type, infrared light is scattered by a grating diffuser and divided into individual frequency lights. In interferometer-type instruments, the infrared light frequencies can interact to produce interference light types. This type is then mathematically analyzed using Fourier transform techniques to obtain individual frequencies and their intensities.
The infrared spectrophotometer consists of the following parts:
â—†Infrared source The main infrared emission source used in the spectrophotometer is:
Nickel-chromium alloy wires protected by ceramic devices; Nernst light emitting elements, ie, a fine filament, containing bonded uranium, thorium, and tantalum oxides; silicon carbon rod filaments.
The above light sources are all electrically heated up to 1200 degrees Celsius to 2000 degrees Celsius. â—† The monochromator was mainly used to split the light scattered by the prism in the early stage, but now it uses a scattering grating. The optical path is dispersed by the reflection of the grating. When the grating is at a specific angle, interference occurs. Therefore, when a special reflection angle is selected, the corresponding interference light is obtained, and a special wavelength is obtained.
Detectors Most scatter instruments use thermopile detectors. These detectors are grouped together by several thermocouples. The sensitivity of the combined output is greatly enhanced. In Fourier Transform Infrared Spectroscopy (FTIR), thermal detectors are mainly composed of pyroelectric materials or solid-state semiconductor devices that use the principle of photo-generated or photo-electrical conduction.
FTIR instruments are generally based on the Michelson interferometer. The beam from the interferometer's infrared light source passes through a 45-degree angle silver mirror to form two beams. The beams thus formed are at right angles to each other. The Michelson interferometer is a very accurate device that can measure changes in length or length through interference fringes.
Advantages of FTIR Compared to conventional infrared (IR), the advantages of FTIR include: high sensitivity; a large improvement in signal-to-noise ratio; easy access to small samples and materials, even if their absorption is very weak The time spent in a full-spectrum spectral scan is no more than one second. This allows multiple scans and average processing of the collected signals to obtain better spectra.
At present, most infrared spectrometers are of the interferometer type (ie, Fourier transform infrared spectrometer). This instrument is widely used for qualitative analysis. But in the rubber and polymer industry, it is used not only for qualitative analysis but also for quantitative analysis.
FTIR applications in the polymer and rubber industries In polymer and rubber production technologies, FTIR is often used to characterize various properties. The following sections describe the application of FTIR in the field of rubber. The instrument used in all analyses was a Model 2000 Fourier Transform Infrared Spectrometer manufactured by Platinum-Almer.
â—† Determination of vinyl acetate content in EVA polyethylene-vinyl acetate In the rubber industry, polyethylene-vinyl acetate is widely used as a packaging material for polymers such as styrene-butadiene rubber, butadiene rubber, etc. Into bags, packing all kinds of rubber chemicals. The properties of EVA polyethylene-vinyl acetate strongly depend on the amount of ethylene acetate content. FTIR can be used to determine the content of ethylene acetic acid in EVA polyethylene-vinyl acetate.
In the infrared spectrum, the absorption peak of the methyl (-CH3) group in ethylene acetic acid is approximately 1370 cm-1, and the absorption band of the vinyl group (-CH2-) in the alkane chain is approximately 720 cm-1. After obtaining the infrared spectrum of the standard substance of known components, the ratio of absorbance near 1370 cm-1 to the absorbance near 720 cm-1 (A1370/A720) is taken as the ratio of ethylene acetate content to ethylene content. The graph will get a straight line. Using the obtained line graph, if the ratio of the absorption peak of the unknown sample (A1370/A720) is known, its ethylene acetate content can also be calculated.
The infrared spectrum of a standard EVA polyethylene-vinyl acetate sample film is baseline-corrected to give an absorbance near 1370 cm-1 and absorbance near 720 cm-1. The clean and transparent EVA polyethylene-vinyl acetate sample film was placed on the electromagnetic sample holder. After the sample holder was loaded with the sample, it was placed in the sample cell for scanning. The spectral wavelength range recorded was 1700-600 cm-1 with a resolution of 4 cm-1. A total of 5 scans were performed and averaged to obtain a better signal-to-noise ratio. In this way, the absorbance near 1370 cm-1 and the absorbance near 720 cm-1 can be calculated from the spectral results, and the obtained values ​​are plotted against the ratio of ethylene acetate content to ethylene content.
From the standard calibration plot we can get the slope M and intercept C of the line, so Y=MX+C
Where Y refers to the ratio of absorbance, X refers to the ratio of ethylene acetate content to ethylene content (A/B). Knowing X(A/B) and having A+B=100, the ethylene acetic acid content can be calculated.
James R. Parker et al. reported on a similar type of application. They used photo-acoustic Fourier transform infrared spectroscopy to quantitatively characterize EVA (polyethylene-vinyl acetate), EPDM (ethylene propylene diene monomer), SBR (styrene-butadiene rubber) and NBR (nitrile-based butadiene rubber), etc. nature. The technique used to analyze samples of a mixture requires first obtaining the background spectrum of the carbon black filler material.
The ASTM D3900 standard provides methods for determining the number of ethylene units in EPM (ethylene propylene rubber) and EPDM (ethylene propylene diene monomer rubber). Thermogravimetric analysis and Fourier transform infrared spectroscopy combined techniques have also been used to quantitatively analyze EVA (polyethylene-vinyl acetate) and NBR (nitrile-based butadiene rubber).
â—† Use FTIR to determine the type of carbon atom in rubber processing oil (CA, CP and CN)
The rubber processing oil used in the rubber industry is mainly mineral oil. This method can be used to determine the type of carbon atoms in a mineral oil (carbon atoms on aromatic rings, carbon atoms on straight chain alkanes, and carbon atoms on cycloalkanes). However, this treatment method is not suitable for water-containing materials. The infrared spectrum records wavelengths in the two regions of 1750 cm-1 to 1500 cm-1 and 859 cm-1 to 600 cm-1.
The intensities of the peaks near 1600 cm-1 and 720 cm-1 can be used to calculate the contents of aromatic ring carbon atoms and straight chain alkanes carbon atoms, respectively. According to the difference, the carbon atoms on the naphthenes are also determined. However, if the content of carbon atoms in the aromatic ring in the mineral oil exceeds 20%, the carbon atoms in the straight chain alkanes cannot be determined directly. Because the peak near the vicinity of 720cm-1 may obscure the absorption peak at 720cm-1. In this case, you need to use dilution techniques to analyze the type of carbon atom.
The length of the light path in the sample cell is determined by the following equation:
d=(n×10)/[2×(w1-w2)]
Where n is the number of gratings between wavelengths w1 and w2.
If there are no gratings, either the cell window is broken or the two lights are not parallel. After baseline correction, the absorbance of the corresponding peak position can be obtained:
CA=1.2+9.8×E
CP=29.9+6.6×E
E = A/c × d where A = absorbance, d = length of light path through the sample cell, and c = concentration factor (when oil is not diluted, c = 1)
CN=100-(CA+CP)
Less than 10% of the results are recorded to one decimal place.
When the content of aromatic rings in the oil is relatively high (20%), it is necessary to add a linear paraffinic mineral oil to dilute the aromatic ring content to 5%.
The CP of the blend can be calculated by the following equation:
CP=CP (blend) (s+d)-CP (diluent) d/s
S = mass of sample in the blend, d = mass of diluent in the blend.
Table 1 records a typical example of the calculation of CA, CP, and CN in linear paraffin oils. From the table below, the CA in linear paraffin oils is relatively low.
Table 1, CA, CP, and CN in Typical Linear Paraffin Oils
Optical path length of the sample cell (d) Wavelength range: 2271cm-1 to 491cm-1, number of gratings: 27d=0.0758mm
Peak CA:1604.22cm-1, Correction Height: 0.0865 Absorbance (A), CA=12.4%
CP peak position: 724.57cm-1, corrected height: 0.3592 absorbance (A), CP = 61.2%
CN value CN=(100-12.4-61.2)%=26.4%
Rubber microstructure determination The Indian Standard IS10016, Section 4 describes the method for determining the microstructure of polybutadiene rubber.
A few drops of polybutadiene rubber were adhered to a KBr transparent wafer to produce a uniform wafer. After drying for 15 to 20 minutes, a test was performed to obtain an infrared spectrum. Absorption at 965 cm-1, 910 cm-1, and 735 cm-1 represents cis, vinyl, and trans absorption peaks, respectively.
After baseline correction, the absorbance at each characteristic wavelength was measured. The relative concentrations of the three components are given by the following equation:
The absorbance of the trans absorption peak at CC = 1 × 735 cm -1 CT = 0.118 × 965 cm -1 The absorbance at the cis absorption peak CV = 0.164 × 910 cm -1 The absorbance of the ethylene absorption peak at the mass percentage of CX = CX×100/(CC+CT+CV)
Table 2 shows a typical example of the calculation of the microstructure of polybutadiene rubber (BR).
Table 2, CC, CT, and CV in Polybutadiene Rubber
Peak value of CC: 739.07cm-1, Correction height: 0.6246 Absorbance (A), CA=0.6246
CT peak position: 967.43 cm-1, corrected height: 0.0269 absorbance (A), CT = 0.0032
Peak CV value: 912.40cm-1, Correction height: 0.0498 Absorbance (A), CT=0.0082
Cis accounted for the total %Cis98.22%
Standard 21561, developed by the International Standards Organization, specifies the determination of the butadiene microstructure and styrene content in styrene-butadiene rubber obtained by solution polymerization. It is based on one-dimensional nuclear magnetic resonance spectroscopy as an absolute method and infrared spectroscopy as a relative method. There have been reports in the literature on the spectroscopic methods for studying the structure of butyl and halobutyl synthetic rubbers. One-dimensional nuclear magnetic resonance spectroscopy was used as an absolute test method to establish a relative method based on Fourier transform infrared spectroscopy. It has also been reported that 6% of isoprene (rubber monomer) is generated by a 1,2-addition reaction using Fourier transform infrared spectroscopy to determine the rubber in butyl and halobutyl synthetic rubbers. Monomer and isoprene content.
â—† Identification of Vulcanized Rubber Polymer-Polymer Blends Due to the differences in the spectral expression patterns, it is strongly recommended that the spectra of a series of reference samples be made on the same machine before an unknown sample is made. Absorption bands shown below are not analytically diagnostic and cannot be used for the identification of rubber: 3330 cm-1, 2861 cm-1, 1700 cm-1, and 1450 cm-1.
The ASTM D 3677 standard specifies the method for the identification of vulcanizates by FTIR. The samples involved are first subjected to pyrolysis. The characteristic absorption bands of some of the listed rubbers can be used as criteria for judgment.
The spectrum of the thermal decomposition products of chloroprene rubber is indeterminate and lacks a significant characteristic absorption value. The most typical is absorption at 820cm-1, but it is usually very wide and the signal is not strong. There is weak absorption at 747cm-1, but it is often not seen, and strong absorption at 885cm-1 is to some extent indistinguishable from other macromolecules. Some of the characteristic absorption bands of the polybutadiene thermal decomposition products are very similar to those of chlorosulfonated polyethylene in both wave number and strength. Therefore, after obtaining the test results of chlorine-containing rubber, if you want to make a choice between two kinds of such rubber, you must carefully consider and consider the test results of chlorine-containing rubber. The main difference between the spectrum of polybutadiene rubber thermal decomposition products and the spectrum of styrene-butadiene rubber thermal decomposition products is the difference in absorption, which is caused by the absence or absence of aromatic functional groups in the former.
IR testing of pyrolysates can also identify mixtures of two rubbers, from 80% of the major components to 20% of the minor components. However, it cannot be distinguished whether it is a microemulsion polymer or a solution polymerized rubber. Similarly, the ratio of acrylonitrile to butadiene could not be measured. This method of testing pyrolyzates cannot distinguish between butyl rubber and its halogenated rubber. In addition, FTIR can not distinguish different levels of fluorine-containing rubber.
If the content of natural and synthetic butadiene in a blend is 20% and the content of chloroprene is 80%, it cannot be identified by this method. Only when the content of a small amount of the component is 30% or more It can only be identified by this method. Similarly, if a styrene butadiene rubber content of 80% and a highly trans-polybutadiene rubber content of 20% in a blend are used, there are also difficulties in this method. Unless a small amount of components reaches or exceeds 30%. If the content of ethylene-propylene rubber in the blend is between 20% and 40%, this method is also not feasible.
O'Keefe reported a good method of acquiring spectral signals that can be used to identify rubber. Frisone and his collaborators reported on the results of their joint laboratories, using a variety of techniques to quantitatively and qualitatively analyze polymer blends, including Fourier transform infrared spectroscopy. Ghebremeskel and his collaborators used infrared spectroscopy to characterize SBR (styrene butadiene rubber), NBR (nitrile butadiene rubber), and PVC (polyvinyl chloride) binary/tertiary blends. Bhatt and his collaborators reported a study of the proportions of polymers in NR (natural rubber)/SBR (styrene-butadiene rubber) and NR (natural rubber)/BR (polybutadiene rubber) blends. The test method used was: Thermogravimetric analysis and FTIR. Differential scanning calorimetry and temperature-dependent FTIR methods were used to study the compatibility of different vinyl content IR (polyisoprene rubber) and BR (polybutadiene rubber) blends.
â—† Identification/characterization of various chemicals in the rubber industry FTIR provides the basis for agreements between producers and consumers. The infrared spectra of the test and reference samples, if they can be coincident, prove that the two are the same material. If the IR spectra do not coincide, there will be a comparison of the position, shape and relative absorbance of each absorption band. If the same material, these conditions will be consistent. The AMS Standard 2702 describes the details of the rubber chemical sampling technique. Some infrared spectra of rubber chemicals are shown in Figure 1 and Figure 2. Figures 1 and 2 are controlled and experimental 6PPD (1,3 dimethylbutyl side-phenylenediamine, used as an antidegradant in the rubber industry). Although they provide relatively clear spectra, there is an option in the software of the FTIR2000 system that can directly overlay or add spectra for comparison. 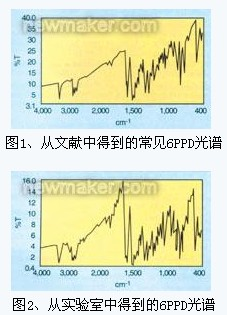
â—† Application of FTIR in other hybrids Gui-Yang Li uses FTIR to describe polymer swelling and solvent phase separation in benzene/cyclohexylamine/polybutadiene rubber systems. The molecular structure of the crack surface in polybutadiene rubber with no silica filled and filled can also be studied by FTIR. Reports of the same type of analysis are also found in the work of Kralevich et al. The materials studied are silica-filled natural rubber mixtures. FTIR has also been used for the study of layered styrene-butadiene rubber nanocomposites. Pcck et al. reported the use of photo-acoustic FTIR for the detection of dispersoids on rubber surfaces.
Conclusion Compared with other technologies, the main advantages of FTIR are: easy operation of the instrument, more economical and feasible operation of the machine, easy interpretation of the data structure, high resolution, rapid time-saving and easy sample preparation. In spite of this, as a relative method, absolute measurement methods are still required to be used in quantitative analysis work. In qualitative analysis, this technique can be well used to identify materials if data are available. Rubber technologists often use it as a tool to solve problems. To date, proper application of FTIR still requires more attention in the rubber field. I believe this technology will have wider application.
Application of Fourier Transform Infrared Spectroscopy FTIR Instrument in Rubber Industry
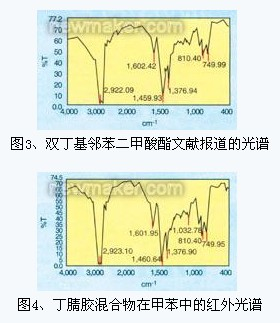
Although almost all bands of electromagnetic light waves in organic chemistry have been used to study the structural properties of materials, our applications have focused on the three or four bands of energy absorption: the ultraviolet and visible light regions (UV-VIS for short); Infrared region (IR); microwave zone; radio frequency absorption zone. Infrared spectroscopy (IR) is basically considered vibrational spectroscopy. The infrared spectrum is divided into three regions: the near infrared region (harmonic region, 12500 ~ 4000cm-1), the mid-infrared region (vibration-rotating region, 4000 ~ 200cm-1) and the far infrared region (rotating region, 200 ~ 10cm- 1). â—† Identification of oil/plasticizers in rubber mixtures The authors used FTIR to characterize the types of oils in rubber mixtures. Using different solvents, the acetone extract is subjected to column chromatography to separate the components. Infrared test was performed on the components separated in toluene. The resulting infrared spectra were compared with the standard spectra in the literature (same experimental conditions). Fig. 3 is a plasticizer dibutyl phthalate, and Fig. 4 is an infrared spectrum of an isolated component of a nitrile rubber (typical oil seal) mixture obtained in toluene. Comparing the two infrared spectra, it can be concluded that the experimental sample is a phthalate type plasticizer. â—†Application of FTIR in the study of reaction mechanism It has been reported that FTIR and NMR were used to study the cross-linking reaction. The reactants were chlorosulfonated polyethylene and epoxidized natural rubber. Thomas and his collaborators studied the mechanism of epoxidation of natural rubber. The same analytical methods used were FTIR and nuclear magnetic resonance spectroscopy. A recent article also reported the use of FTIR to study the curing reaction of brominated poly(isobutylene-4-methylstyrene), and in this way, a number of different zinc salts that can be used for curing reactions were discovered. FTIR was also used to determine the remaining amount of vulcanization accelerator in the latex film. FTIR has also been used to study various reaction mechanisms. SungJoonOh used FTIR to study the peroxide curing mechanism of polybutadiene and zinc diacrylate. FTIR has also been used to study the thermal autoxidation of trans-1,4-polybutadiene at different temperatures.